Mechanism of Enzyme Reaction
Any two molecules have to collide for the reaction to occur along with the right orientation and a sufficient amount of energy. The energy between these molecules needs to overcome the barrier in the reaction. This energy is called activation energy.
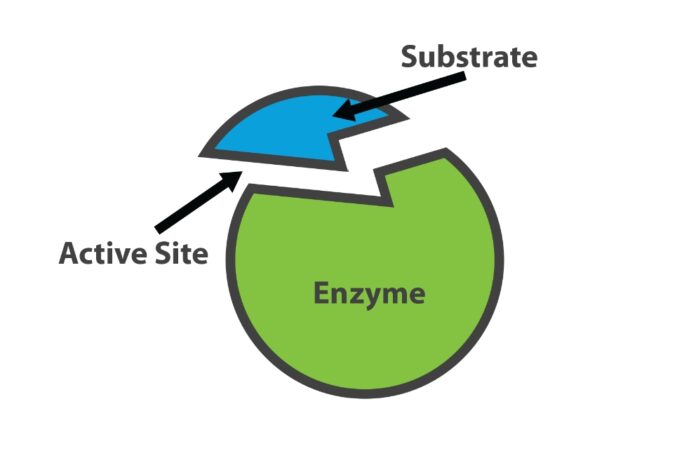
Enzymes are said to possess an active site. The active site is a part of the molecule that has a definite shape and the functional group for the binding of reactant molecules. The molecule that binds to the enzyme is referred to as the substrate group. The substrate and the enzyme form an intermediate reaction with low activation energy without any catalysts.
Reactant1 + Reactant2 = Product
Reactant1 + enzyme = Intermediate
Intermediate + Reactant2 = Product + Enzyme
The basic mechanism of enzyme action is to catalyze the chemical reactions, which begins with the binding of the substrate with the active site of the enzyme. The enzyme’s active site is a cleft or a pocket within the enzyme where the substrate molecule binds and undergoes chemical reactions to be converted into the product.
Enzyme-Substrate Interactions
Enzymes are biocatalysts, which are high molecular weight proteinous compounds.
It helps the substrate by providing the surface for the reaction to occur.
The enzyme comprises hollow spaces occupying groups such as -SH, -COOH, and others on the outer surface.
The substrate which has an opposite charge of the enzyme fits into these spaces, just like a key fits into a lock. This substrate binding site is called the active site of an enzyme (E).
The favourable model of enzyme-substrate interaction is called the induced-fit model. This model states that the interaction between substrate and enzyme is weak, and these weak interactions induce conformational changes rapidly and strengthen binding and bring catalytic sites close enough to substrate bonds.
There are four possible major mechanisms of catalysis:
Catalysis by Bond Strain
The induced structural rearrangements in this type of catalysis produce strained substrate bonds that attain transition state more easily. The new conformation forces substrate atoms and catalytic groups like aspartate into conformations that strain substrate bonds.
Covalent Catalysis
The substrate is oriented to active place on the enzymes in such a manner that a covalent intermediate develops between the enzyme and the substrate, in catalysis that occurs by covalent mechanisms. The best example of this involves proteolysis by serine proteases that have both digestive enzymes and various enzymes of the blood clotting cascade. These proteases possess an active site serine whose R group hydroxyl generates a covalent bond with a carbonyl carbon of a peptide bond and results in the hydrolysis of the peptide bond.
Catalysis Involving Acids and Bases
Other mechanisms add to the completion of catalytic events which are launched by strain mechanisms such as the usage of glutamate as a general acid catalyst.
Catalysis by Orientation and Proximity
Enzyme-substrate interactions induce reactive groups into proximity with one another. Also, groups like aspartate are chemically reactive, and their proximity towards the substrate favours their involvement in catalysis.
Action and Nature of Enzymes
Once substrate (S) binds to this active site, they form a complex (intermediate-ES) which then produces the product (P) and the enzyme (E). The substrate which gets attached to the enzyme has a specific structure and that can only fit in a particular enzyme. Hence, by providing a surface for the substrate, an enzyme slows down the activation energy of the reaction. The intermediate state where the substrate binds to the enzyme is called the transition state. By breaking and making the bonds, the substrate binds to the enzyme (remains unchanged), which converts into the product and later splits into product and enzyme. The free enzymes then bind to other substrates and the catalytic cycle continues until the reaction completes.
The enzyme action basically happens in two steps:
Step1: Combining of enzyme and the reactant/substrate.
E+S → [ES]
Step 2: Disintegration of the complex molecule to give the product.
[ES]→E+P
Thus, the whole catalyst action of enzymes is summarized as:
E + S → [ES] → [EP] → E + P
Biological Catalysts
Catalysts are the substances which play a significant role in the chemical reaction. Catalysis is the phenomenon by which the rate of a chemical reaction is altered/ enhanced without changing themselves. During a chemical reaction, a catalyst remains unchanged, both in terms of quantity and chemical properties. An enzyme is one such catalyst which is commonly known as the biological catalyst. Enzymes present in the living organisms enhance the rate of reactions which take place within the body.
Biological catalysts, enzymes, are extremely specific that catalyze a single chemical reaction or some closely associated reactions. An enzyme’s exact structure and its active site decide an enzyme’s specificity. Substrate molecules attach themselves at the active site of an enzyme. Initially, substrates associate themselves by noncovalent interactions to the enzymes which include ionic, hydrogen bonds and hydrophobic interactions. Enzymes reduce the reactions and activation energy to progress towards equilibrium quicker than the reactions that are not catalyzed. Both eukaryotic and prokaryotic cells usually make use of allosteric regulation to respond to fluctuations in the state inside the cells.